Introduction
Glycogen synthase kinase 3 (GSK3), a ubiquitously expressed serine/threonine kinase, is considered a therapeutic target in non-Hodgkin lymphomas (NHL) due to the poorer survival of patients with higher tumor expression, and the previously observed lethality of GSK3 ‘knock-out’ lymphoma cell lines. Elraglusib (formerly 9-ING-41), a small molecule reported to inhibit the GSK3β paralog, reduces proliferation and induces apoptosis in NHL cell lines, and is undergoing early-phase clinical trials (Wu et al, Blood 2019). However, in recent cell culture studies, the anti-lymphoma effects of elraglusib could not be phenocopied by structurally unrelated, small-molecule GSK3 inhibitors, raising questions about the importance of GSK3-dependent pathways in lymphoma biology and treatment (Coats et al, Cell Commun. Signal 2023). To further validate the lack of impact of GSK3 reduction on lymphoma viability and improve our understanding of the pharmacological properties of elraglusib, we have investigated the effects of reducing GSK3α, GSK3β, or both paralogs concomitantly in lymphoma cells, using paralog-specific and dual GSK3A/B genetic ablation techniques. Additionally, we performed cell-free kinase assays to explore the relative sensitivity of GSK3α and GSK3β as targets of elraglusib.
Methods
In the lymphoma cell line Karpas-299 (K299), single paralog GSK3A and GSK3B knockdowns (KD) were generated using commercial lentiviral shRNA transfection with subsequent puromycin-selection. Dual paralog KD (both GSK3A and GSK3B)were generated by transfecting stable GSK3A KD cells with a second commercial GSK3B lentiviral shRNA and subsequent neomycin-selection. KD efficiency was quantified by total-GSK3 immunoblot and apoptosis by poly (ADP-ribose) polymerase (PARP) cleavage immunoblot. Cell viability and proliferation were assessed by MTS assay following 72-hour incubations in complete media (RPMI 1640, 10% FBS, 100units/ml penicillin/100µg/ml streptomycin). Paralog-specific cell-free kinase assays were performed using 32P-γ-ATP, phospho-glycogen synthase-peptide-2 substrate and purified recombinant GSK3α and GSK3β.
Results
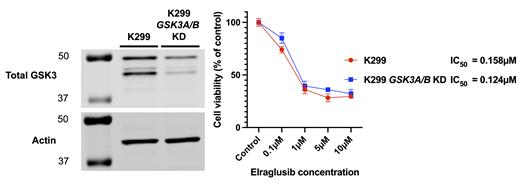
Viable and stable K299 single-paralog ( GSK3A or GSK3B) and dual-paralog ( GSK3A and GSK3B) KD cell lines were obtained. KD efficiency was quantified by immunoblot and compared to the parental cell line control for GSK3α (≈30% of control), GSK3β (≈20% of control) and dual GSK3αand GSK3β KD (≈35% control and ≈15% control respectively). All KD cell lines proliferated similarly to parental cell line cells by MTS assay (control vs GSK3B KD normalized to control, mean difference -5.1% (95% CI -11.7 to +1.37 p=0.14), control vs GSK3A/B KD +3.67% (95% CI -2.87 to +10.2 p=0.348)). Elraglusib 5µM induced apoptosis with a significant increase in cleaved/intact PARP (2.35x, p<0.0001) and reduced cell viability in K299 parental cell line and dual GSK3A/B KD cells (IC 50 0.158µM parental control vs 0.124µM dual GSK3A/B KD). The equivalence of paralog inhibition (GSK3β IC 50 0.494µM and GSK3α IC 50 0.446µM) was confirmed in cell-free 32P-γ-ATP kinase assays.
Conclusion
K299 cells do not depend upon high levels of GSK3 for survival or proliferation. This data extends previous findings regarding the redundancy of GSK3-dependent signalling in lymphoma biology and further suggests that limited therapeutic benefit may be derived from drugs specifically targeting the activity or expression of this protein kinase. The IC 50 of elraglusib remains similar despite a significant reduction in levels of GSK3, again suggesting that GSK3 is not its cytotoxic target. Importantly, elraglusib retains therapeutic potential in lymphoma however GSK3 is not a surrogate marker for its efficacy and additional studies are required to elucidate the mechanisms through which it mediates its cytotoxic effects.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal